
The LESA Plant Science team is pursuing research activities on the impacts of improved horticultural lighting, sensing and control systems for Controlled Environment Agriculture (CEA) including greenhouses and vertical farms. By blending research in applied engineering and plant physiology, our research is aimed at improving energy efficiency and increasing crop yield while improving plant nutritional content.
Our current projects include research on the effect of blue spectral changes on the growth of lettuce, tomato and strawberry in indoor growth environments (GLASE project). We are also interested in how high-frequency pulsed LED lighting can be used to save electricity in vertical farms in addition to the interplay between daylength and daylight integral for crop growth control. On the sensor side, we are studying chlorophyll a fluorescence and phytoelectrical signaling in response to changes in horticultural LED lighting.
People
- Elsebeth Kolmos, Senior Research Scientist
- Rick Neal, Research Engineer
- Lily K. Donaldson, Graduate Student
- Jadyn Ide-Pech, URP Undergraduate Student
- Eurydice Molina, Undergraduate Intern
- Sarah-Helen Mysliwiec, Student Intern, Hoosic Valley Central School
Collaborators
- Neil Mattson, Cornell University/GLASE
- AJ Both, Rutgers University/GLASE
- Mattheos Koffas, RPI
- Marianne Nyman, RPI
- Josh Draper, CASE, RPI
Current Projects
Blue spectral quality for indoor growth of strawberry
We are testing the role of blue light for ‘Albion’ strawberry growth and yield under sole-source LED lighting, similar to previous projects involving lettuce and tomato (see below).

GHL2 Greenhouse Light
We are designing a horticultural LED luminaire modeled after the GE Arize Light fixture with four independently controllable wavelengths (450 nm, 660 nm, 735 nm, and white light with an undetermined CCT). The design, based on the 48” GE Arize fixture previously tested at LESA and Rutgers, will use standard LEDs sourced from various vendors for optimal performance and cost-effectiveness. The fixture's drivers and power supplies will be housed separately for ease of design, with each light channel being dimmable from 5% to 100% and programmable via a simple GUI. The GUI, adapted from the existing TIGER lighting system, will allow independent adjustments of blue/red and red/far-red ratios, white-to-monochromatic PPFD ratios, and channel intensity scheduling, in addition to a wireless interface.
PlantPulse chlorophyll a fluorescence sensor
 PlantPulse is a remote fluorometer designed to measure chlorophyll a fluorescence from a distance, enabling the assessment of plant health by analyzing photosynthetic efficiency, stress levels, and overall vitality. This compact, user-friendly device can capture signals <30 cm above the leaf or canopy, facilitating automated and non-invasive real-time monitoring. Aimed at CEA professionals, plant scientists, and sustainability-focused researchers, PlantPulse offers crucial, field-friendly insights without the need for extensive technical expertise. The device features bright LEDs that excite chlorophyll molecules and a sensor that detects emitted fluorescence, with data being transmitted wirelessly to a collection node for analysis and display. We have successfully tested prototypes in the lab, where consistent data has been collected from tobacco plants under controlled light and watering conditions. The data recorded reflect environmental conditions, with changes in fluorescence correlating with chlorophyll levels, such as those observed in aging leaves. The ability of PlantPulse to provide reliable, real-time insights will help CEA growers to manage plant health proactively, optimize resources, and improve yield.
PlantPulse is a remote fluorometer designed to measure chlorophyll a fluorescence from a distance, enabling the assessment of plant health by analyzing photosynthetic efficiency, stress levels, and overall vitality. This compact, user-friendly device can capture signals <30 cm above the leaf or canopy, facilitating automated and non-invasive real-time monitoring. Aimed at CEA professionals, plant scientists, and sustainability-focused researchers, PlantPulse offers crucial, field-friendly insights without the need for extensive technical expertise. The device features bright LEDs that excite chlorophyll molecules and a sensor that detects emitted fluorescence, with data being transmitted wirelessly to a collection node for analysis and display. We have successfully tested prototypes in the lab, where consistent data has been collected from tobacco plants under controlled light and watering conditions. The data recorded reflect environmental conditions, with changes in fluorescence correlating with chlorophyll levels, such as those observed in aging leaves. The ability of PlantPulse to provide reliable, real-time insights will help CEA growers to manage plant health proactively, optimize resources, and improve yield.
Phytoelectrical signaling
Plants generate electrical signals that can be measured and that induce physiological changes in the plant. These signals occur in response to both biotic and abiotic stressors and have both non-specific and stimulus-specific expressions. At LESA, we are studying phytoelectric profiles of plants under various lighting conditions, including pulsed lighting, using a Vivent PhytlSigns plant electrophysiology measurement device.
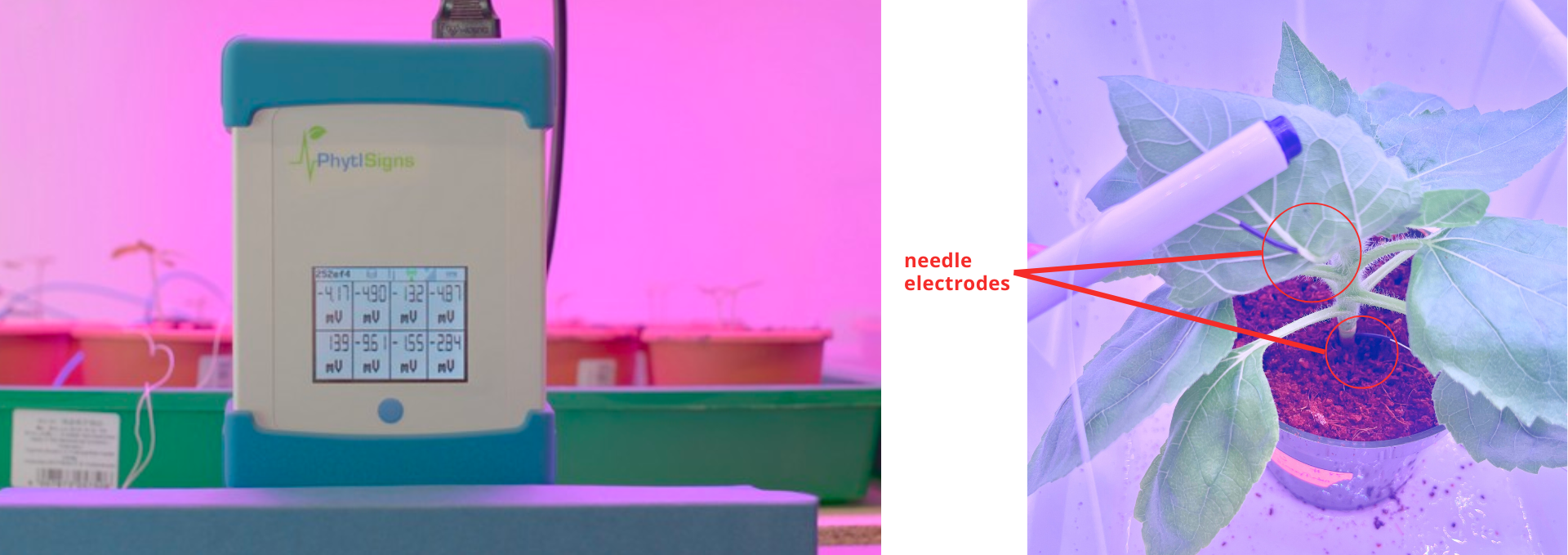
Pulsed LED lighting
Pulsed lighting is the process of modulating light over time, often to achieve a mean output that is similar to continuous light. Pulsed lighting may improve plant outcomes (including increased biomass, photosynthetic rate, pigment concentration, and antioxidant response) and therefore reduce energy requirements within Controlled Environment Agriculture (CEA) applications. There are three main variables for pulsed lighting: duty cycle, frequency, and intensity. Duty is a measurement of tempo that counts the percentage of time that the light is on for a given duty cycle time period. Frequency is how quickly the duty cycles occur, and intensity measures the amplitude of the wave, or how intense the light is during each pulse. By varying these variables of pulsed lighting, lower energy use and improved crop outcomes may be achieved.
Interaction of photoperiod and DLI for microgreen growth
The stem growth of seedlings (microgreens) is often controlled by both the daylength and the light intensity. We are testing the interaction of photoperiod and daylight integral during growth of different species of microgreens with the aim to define the most energy efficient protocols for electrical lighting.
Past Projects
TIGER lights
 GLASE researchers developed TIGER LED light fixtures for tunable irradiance growth efficiency research, tailored for controlled environment plant growth studies. The LED lights provide flexibility in wavelength selection, high PPFD, uniform color mixing and canopy lighting, and a modular design for various cabinet types. The system, comprising six LED wavelengths (400 nm, 420 nm, 450 nm, 525 nm, 660 nm, and 735 nm), avoids phosphor-converted LEDs for precise wavelength control. Custom 6-in-1 LED packages ensure excellent color mixing without a diffuser. Each module, capable of pulsed and analog dimming, is equipped with six calibrated drivers for independent wavelength control, enabling high-speed operation for potential energy-efficient lighting protocols to enhance plant growth and biomass efficacy.
GLASE researchers developed TIGER LED light fixtures for tunable irradiance growth efficiency research, tailored for controlled environment plant growth studies. The LED lights provide flexibility in wavelength selection, high PPFD, uniform color mixing and canopy lighting, and a modular design for various cabinet types. The system, comprising six LED wavelengths (400 nm, 420 nm, 450 nm, 525 nm, 660 nm, and 735 nm), avoids phosphor-converted LEDs for precise wavelength control. Custom 6-in-1 LED packages ensure excellent color mixing without a diffuser. Each module, capable of pulsed and analog dimming, is equipped with six calibrated drivers for independent wavelength control, enabling high-speed operation for potential energy-efficient lighting protocols to enhance plant growth and biomass efficacy.
IRGA LED attachment
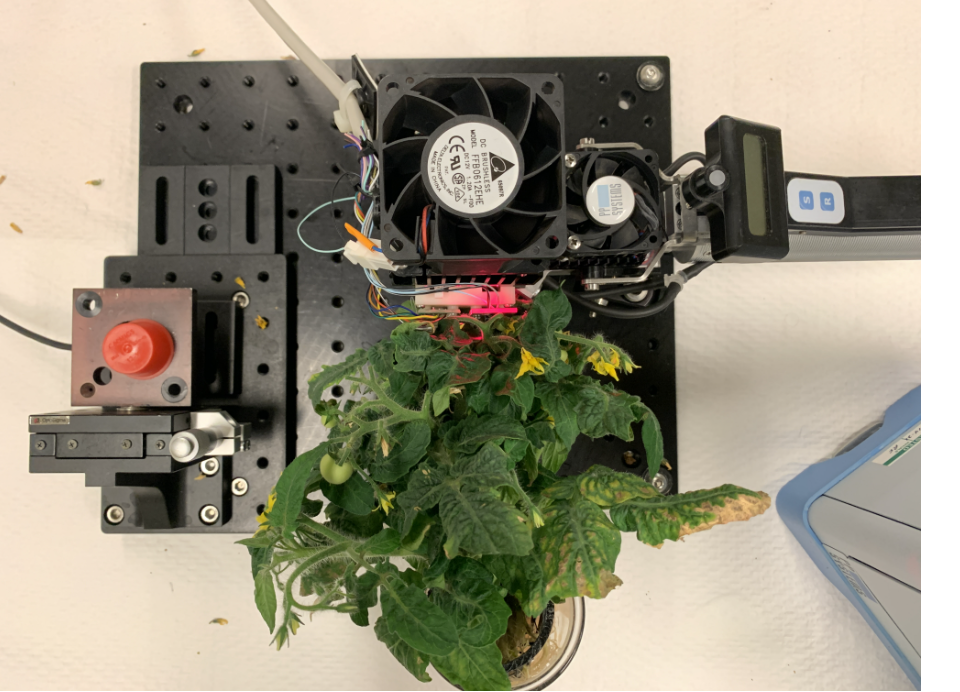 Plants rely on sunlight for energy, where photosynthesis converts carbon dioxide (CO2) and water (H2O) into oxygen (O2) and sugar. The infra-red gas analyzer (IRGA) is a tool used to measure photosynthetic rates by detecting gas absorption, specifically CO2 and H2O. At LESA we use the IRGA enhanced with a custom LED module, termed TIGER Cub. This module, adapted from the TIGER horticultural research LED fixture, includes six adjustable LED channels (three blue, one green, one red, and one far-red) for accurate simulation of growth chamber lighting conditions. The TIGER Cub is placed on the IRGA leaf chamber, facilitating precise photosynthetic studies under controlled light spectra including photosynthetic response analysis, spectral testing for crop growth, and high-frequency pulsed lighting studies. This setup enables real-time and direct comparisons of plant responses to various light conditions, aiding in the development of optimized growth lighting strategies.
Plants rely on sunlight for energy, where photosynthesis converts carbon dioxide (CO2) and water (H2O) into oxygen (O2) and sugar. The infra-red gas analyzer (IRGA) is a tool used to measure photosynthetic rates by detecting gas absorption, specifically CO2 and H2O. At LESA we use the IRGA enhanced with a custom LED module, termed TIGER Cub. This module, adapted from the TIGER horticultural research LED fixture, includes six adjustable LED channels (three blue, one green, one red, and one far-red) for accurate simulation of growth chamber lighting conditions. The TIGER Cub is placed on the IRGA leaf chamber, facilitating precise photosynthetic studies under controlled light spectra including photosynthetic response analysis, spectral testing for crop growth, and high-frequency pulsed lighting studies. This setup enables real-time and direct comparisons of plant responses to various light conditions, aiding in the development of optimized growth lighting strategies.
SASSy
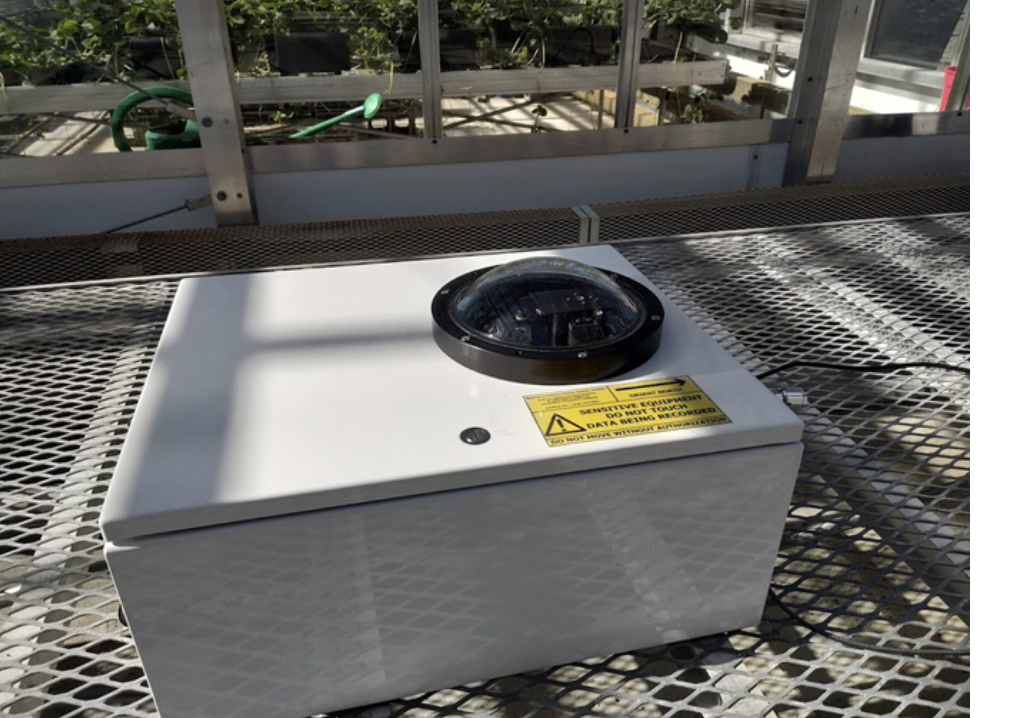 The Spectral Acquisition Sensor System (SASSy) was developed to monitor and record changes in the solar spectrum throughout the day and across seasons, which is essential for optimizing light conditions for plant growth. SASSy captures visible light spectra from 350 to 820 nm every 20 to 30 seconds and compensates for detector drift with an integrated shutter. This system outperforms traditional daily light integral (DLI) measurements by providing continuous spectral data, enabling precise adjustments to greenhouse lighting conditions. Initial data showed significant variability due to factors like cloud cover, which can impact plant growth. The collected spectral data helps to understand and predict light variations and informs adaptive lighting systems that stabilize light conditions. The integration of SASSy with Cornell University's Lighting and Shade System Implementation (LASSI) will allow for dynamic control using tunable LEDs and shades to maintain optimal lighting, ensuring consistent and high-quality crop growth.
The Spectral Acquisition Sensor System (SASSy) was developed to monitor and record changes in the solar spectrum throughout the day and across seasons, which is essential for optimizing light conditions for plant growth. SASSy captures visible light spectra from 350 to 820 nm every 20 to 30 seconds and compensates for detector drift with an integrated shutter. This system outperforms traditional daily light integral (DLI) measurements by providing continuous spectral data, enabling precise adjustments to greenhouse lighting conditions. Initial data showed significant variability due to factors like cloud cover, which can impact plant growth. The collected spectral data helps to understand and predict light variations and informs adaptive lighting systems that stabilize light conditions. The integration of SASSy with Cornell University's Lighting and Shade System Implementation (LASSI) will allow for dynamic control using tunable LEDs and shades to maintain optimal lighting, ensuring consistent and high-quality crop growth.
GHL1 Greenhouse Light
The GHL1 light is spectrally tunable with six adjustable LED channels (405 nm, 423 nm, 450 nm, 525 nm, 660nm, and 735 nm) that enable direct comparison with the TIGER lights. Each light has three arrays, each with four lights, when mounted in a 1.2 x 1.2 m grid with adjustable chain height can illuminate a 4’ x 4’ area. GHL1 mixes colors effectively without a diffuser and at a close distance show individual colors, but white light appears at the plant canopy. The adjustable light spectrum and intensity is a valuable tool for both research and student projects.

Lettuce response to blue light quality
 We tested lettuce growth following modifications in blue LED light quality. Monochromatic blue light responses showed that net photosynthesis (Pn) for 420 nm and 450 nm was quite similar, while 400 nm significantly lowered Pn. When blue light was combined with red and far-red light, the differences in Pn among the three blue wavelengths became minimal for the ‘Green Star’ cultivar. In the ‘Red Sails’ cultivar, 400 nm in the mixed spectrum led to significant reduction in Pn, particularly at lower light intensities. Growth under the various blue LED wavebands combined with red and far-red light showed minimal phenotypic differences. There was no significant blue light effect on biomass, though chlorophyll a fluorescence indicated reduced photosynthetic efficiency for spectra with 400 nm and 420 nm compared to 450 nm. The spectrum with 450 nm increased chlorophyll content index and chlorophyll a/b ratio, while spectrum with 400 nm only caused a slight decrease in violaxanthin pigment in terms of carotenoid content. Thus, lettuce can detect changes in the blue light spectrum affecting photosynthetic efficiency and chlorophyll composition, but overall growth and yield remain largely unchanged.
We tested lettuce growth following modifications in blue LED light quality. Monochromatic blue light responses showed that net photosynthesis (Pn) for 420 nm and 450 nm was quite similar, while 400 nm significantly lowered Pn. When blue light was combined with red and far-red light, the differences in Pn among the three blue wavelengths became minimal for the ‘Green Star’ cultivar. In the ‘Red Sails’ cultivar, 400 nm in the mixed spectrum led to significant reduction in Pn, particularly at lower light intensities. Growth under the various blue LED wavebands combined with red and far-red light showed minimal phenotypic differences. There was no significant blue light effect on biomass, though chlorophyll a fluorescence indicated reduced photosynthetic efficiency for spectra with 400 nm and 420 nm compared to 450 nm. The spectrum with 450 nm increased chlorophyll content index and chlorophyll a/b ratio, while spectrum with 400 nm only caused a slight decrease in violaxanthin pigment in terms of carotenoid content. Thus, lettuce can detect changes in the blue light spectrum affecting photosynthetic efficiency and chlorophyll composition, but overall growth and yield remain largely unchanged.
Tomato response to blue light quality
 We compared the effects of different blue LED wavebands (400 nm, 420 nm, and 450 nm) on ‘Micro Tom’ tomato growth and yield under sole-source lighting. We conducted a comparative analysis of photosynthetic CO2 assimilation under mono- and poly-chromatic light following changes in the blue wavebands; second, we evaluated tomato growth and yield under poly-chromatic light with similar blue spectral differences. We found that only net photosynthetic rate, not photochemical efficiency, was affected by variations in blue light quality when measured at the single leaf level. Tomato growth and yield, however, was more sensitive to blue light quality than photosynthesis. Shorter wavelength blue light promoted larger leaf size, while longer blue wavelengths increased biomass and the fruit-to-leaf ratio (harvest index). Specifically, blue light at 450 nm was linked to a lower chlorophyll a/b ratio and higher ascorbic acid (vitamin C) content in the fruits. Overall, blue spectral quality had a subtle impact on leaf photochemistry but a more significant effect on tomato crop physiological outcomes.
We compared the effects of different blue LED wavebands (400 nm, 420 nm, and 450 nm) on ‘Micro Tom’ tomato growth and yield under sole-source lighting. We conducted a comparative analysis of photosynthetic CO2 assimilation under mono- and poly-chromatic light following changes in the blue wavebands; second, we evaluated tomato growth and yield under poly-chromatic light with similar blue spectral differences. We found that only net photosynthetic rate, not photochemical efficiency, was affected by variations in blue light quality when measured at the single leaf level. Tomato growth and yield, however, was more sensitive to blue light quality than photosynthesis. Shorter wavelength blue light promoted larger leaf size, while longer blue wavelengths increased biomass and the fruit-to-leaf ratio (harvest index). Specifically, blue light at 450 nm was linked to a lower chlorophyll a/b ratio and higher ascorbic acid (vitamin C) content in the fruits. Overall, blue spectral quality had a subtle impact on leaf photochemistry but a more significant effect on tomato crop physiological outcomes.
High-frequency pulsed lighting
We tested the impact of multi-color pulsed LED irradiation on the growth, photosynthetic efficiency, and antioxidant properties of baby-leaf lettuce 'Defender'. The study compared the effects of continuous and pulsed lighting (0.5 kHz and 1 kHz with a 50% duty cycle) using blue (450 nm), green (520 nm), red (660 nm), and far-red (735 nm) light components, while keeping the total daily light integral (DLI; 14.4 mol m−2 day−1) constant over a 16-hour photoperiod. Our results indicated that lettuce grown under pulsed light exhibited superior growth, with notable increases in fresh weight, dry weight, and leaf area. Both pulsed treatments enhanced photosynthetic rate and chlorophyll and carotenoid content. However, the total phenol content and antioxidant activity depended on the specific pulsed light frequency, with the 1 kHz treatment being the most effective for boosting phenol levels and antioxidant activity. Our findings highlight the potential of high-frequency pulsed LED lighting to enhance photosynthetic performance and antioxidant properties in lettuce grown indoors. Future electrical lighting systems in CEA might be able to optimize energy use by integrate pulsed lighting techniques to produce high-quality crops.
Plant Science Publications
Gil-Marin, J., Goldman, M. S., & Kolmos, E. 2023. Changes in amaranth microgreen growth in response to differing light conditions. Journal of Student Research 12:4.
Goldman, M. S., & Kolmos, E. 2023. Blue spectral quality contributes to yield and ascorbic acid content in Micro Tom tomato under sole-source lighting. bioRxiv. doi: 10.1101/2023.11.27.568869
Karlicek, R.F. & Neal, R.R. 2021. Horticulture lighting fixture thermal performance. GLASE Technical Article 9.
Kolmos, E., Neal, R.R., Tuzikas, A. & Karlicek, R.F. 2021. Infra-red gas analyzer with custom LED module attachment for multi-spectral photosynthetic analysis. GLASE Technical Article 7.
Miliauskienė, J., Karlicek, R.F. & Kolmos, E. 2021. Effect of multispectral pulsed light-emitting diodes on the growth, photosynthetic and antioxidant response of baby leaf lettuce (Lactuca sativa L.). Plants, 10, 762. doi: 10.3390/plants10040762
Kolmos, E., Neal, R.R. & Karlicek, R.F. 2020. Greenhouse solar spectral measurements offer powerful future farming tool. GLASE Technical Article 4.
Karlicek, R.F. 2019. New research LED fixture for plant growth. GLASE Technical Article 1
2018
M.R. Urschel and T. Pocock, “Remote Detection of Growth Dynamics in Red Lettuce Using a Novel
Chlorophyll a Fluorometer”, Agronomy, 8(10), 227, (2018).
R. Huang, T. Pocock, Z. Deng, and N.S. Mattson, “Effect of Light Spectrum on Pigment Accumulation and
Expression of Pigment Biosynthesis Genes in Red Leaf Lettuce”, ASHS Annual Conference, (2018).
A.M. Srivastava, M.G. Brik, W.W. Beers, T. Pocock and H.A. Comanzo, “Spectroscopy of Mn4+ activated
Perovskites, LA2LiSbO6 and La2MgTiO6: Hosts for deep red photons for agriculture LEDs”, ECS J. Solid
State Sci. Technol, Vol 7, Issue 1, R3158-R3162, (2018).
2017
T. Pocock, “Influence of Light Emitting Diodes (LEDs) on light sensing and signaling networks in plants”,
Light Emitting Diodes (LEDs) in Agriculture. In: Dutta Gupta S. (eds) Light Emitting Diodes for Agriculture.
Springer, Singapore, (2017).
T. Pocock, “The Biological Effects of Different Phosphors Used in LED Down Conversion”, ASHS Annual
Conference, (2017).
2016
T. Pocock, “Going Beyond Growth With Horticultural Lighting,” Proceeding of 15th International
Symposium on Science and Technology of Lighting, Kyoto Japan, (May 22-27, 2016).
LESA Jan 2023
T. Pocock, “Advanced Lighting Technology in Controlled Environment Agriculture,” Lighting Research and
Technology, Vol. 48, pp. 83-94, (February 2016).
A-M Carstensen, T. Pocock, D. Bånkestad, and T Wik, “Remote Detection Of Light Tolerance In Basil
Through Frequency And Transient Analysis Of Light Induced Fluorescence,” Computers and Electronics in
Agriculture. 127: 289-301 (2016).
2015
T. Pocock, “Light-Emitting Diodes And The Modulation Of Specialty Crops: Light Sensing And Signaling
Networks In Plants,” HortScience, Vol. 50(9), pp. 1281-1284 (2015).
Student Alumni
Graduate Students
- Will Pepi, 2021-2024
Pepi currently leads a startup called Biom which is based on concepts from his Ph.D. research (LESA advisors Karlicek and Kolmos) which was a collaboration involving RPI’s Center for Architectural Science and Ecology (CASE) in the School of Architecture and the Center for Lighting Enabled Systems and Applications (LESA).
Undergraduate Research Projects
- Hui Min Guo, URP 2022
- Muyuan Tian, URP 2022
- Cassandra Shore, URP 2022-2023
- Jasmine King, URP 2023
- Corinne Wingrove, URP 2023-2024
RPI STEP
- Julieta Gil-Marin, STEP Student 2022-2023
- Edward Boakye, STEP Student 2023-2024
- Hadeel Hamed, STEP Student 2023-2024